

Many birds stand on one leg when resting on the ground (Clark 1973, Stiefel 1979;
Necker 2010; see Liste). This behavior can best be observed in long-
Functional Anatomy
Keeping balance in a biped vertebrate
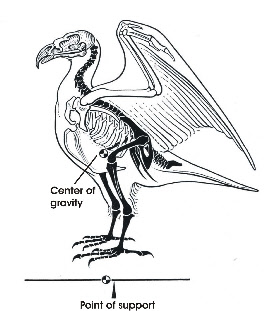
Bipedal locomotion is found in humans and in birds. In humans the body is oriented vertically, i.e. in line with the line of gravity, and the center of gravity is located near the insertion of the legs. In birds the body is oriented more horizontally and the center of gravity is located rostral to the insertion of the hindlimbs (Fig. 1). This imposes special demands on keeping balance.
Birds standing on one leg: mechanisms and meaning
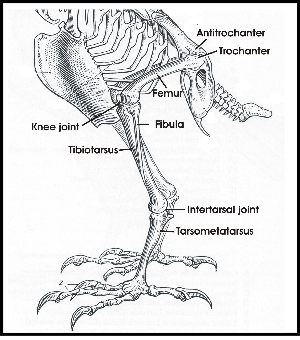
The legs of birds are different from bipedal humans in that they walk on their toes and there is an ankle joint which looks like the human knee but with a reversed angle. There is an upper leg with a femur and a lower leg with a tibia and a fibula. Upper leg and lower leg are connected by a knee joint (Fig. 2). The distal end of the tibia includes parts of tarsal bones. Therefore the lower leg is called tibiotarsus. The remaining tarsal elements fuse with metatarsal bones to make up the tarsometatarsus or tarsus (Baumel & Witmer 1993). The tarsus looks like a lower leg and this impression is furthered by the fact that upper and lower legs are normally hidden in the plumage. Tibiotarsus and tarsometatarsus are connected by the intertarsal joint (Fig. 2).
To keep balance when standing on both legs, the knees are flexed, which puts the knee joint near the center of gravity (Fig. 1). The antitrochanter (Fig. 2), a structure unique to birds, forms a joint with the trochanter of the femur which serves to prevent abduction of the femur and to absorb stresses which would otherwise act on the head of the femur (Hertel and Campbell 2007). The feet are positioned under the center of gravity which results in a stable balance of the body.
When resting on both legs, the femur is oriented nearly horizontally. A further unintended upward movement of the femur is prevented mechanically by the antitrochanter and the ligaments of the hip joint. The hip joint is now in a fixed position. Because of the high position of the knee, the center of gravity may shift to below the knee. This means that the body is suspended at the knee joint, which results in a very stable position that does not need much muscle activity to keep balance.
Standing on one leg
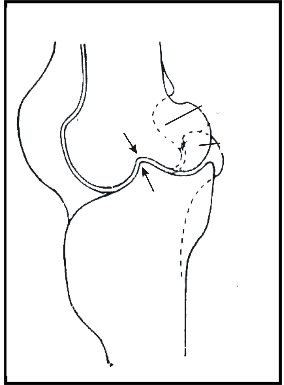
When resting, the supporting foot is set below the center of gravity (Figs. 3, 4). This position stresses the lateral ligaments of both knee and intertarsal joints (see arrows) but these ligaments are well developed in birds.
Long-
In long-
Conclusions
Most birds stand or sleep on one leg without having specializations in their legs.
The leg is positioned in such a way that the body is well balanced without much additional
muscle activity. Most long-
A useful function of standing on one foot with hiding the non-
Literature:
Anderson MJ (2016) Flamingo resting behavior: a general description and sampling of findings. In: Flamingos. Behavior, Biology and Relationship with Humans. Nova Science publishers 2016.
Anderson MJ, Williams SA (2009) Why do flamingos stand on one leg? Zoo Biology 28.:1-
Anderson MJ, Laughlin CP (2014) Investigating laterality, social behavior, and temperature
effects in captive Chilean flamingos, Phoenicopterus chilensis. Avian Ecol. Behav.
25:3-
Anderson MJ, Jones AG, Schlosnagle AP, King ML, Perretti A (2018): Examining unihemispheric
sleep and its potential relation to lateral resting behaviour and unipedal resting
stance in Caribbean Flamingos. Avian Biol Res 11: 74-
Baumel JJ, Witmer LM (1993) Osteologia. In: Handbook of avian anatomy: Nomina anatomica
avium. 2nd edition: 45-
Bell C (1847) Die Hand und ihre Eigenschaften (translated from the English by F Kottenkamp; original: The hand, its mechanism and vital endowment as evincing design). Expedition der Wochenbände, Stuttgart
Bouchard LC, Anderson MJ (2011) Caribbean Flamingo resting behavior and the influence
of weather variables. J Ornithol 152: 307-
Chang Y-
Clark GA 1973 Unipedal postures in birds. Bird Banding 44: 22-
Dawson WR, Whittow GC (2000) Regulation of body temperature. In: Whittow GC (Hrsg)
Sturkies´s Avian Physiology: 343-
Flamingo file (1991): New Scientist Nr. 1782 vom 17. August 1991, Letters: Flamingo file. URL: http://www.newscientist.com/search?doSearch=true&query=Flamingo+file.
Harker TD, Harker FR (2010) Why do birds stand on on leg? -
Hertel F, Campbell KE Jr (2007) The antitrochanter of birds: form and function in
balance. Auk 124: 789-
Kahl MP Jr (1963) Thermoregulation of the wood stork, with special reference to the
role of the legs. Physiol Zool 36: 141-
Herzog K (1968) Anatomie und Flugbiologie der Vögel. Fischer, Stuttgart.
Langer K (1859) Über die Fussgelenke der Vögel. Zweiter Bericht. Zur vergleichenden
Anatomie und Mechanik der Gelenke. Denkschriften der kaiserlichen Akademie der Wissenschaften,
mathematisch-
McFarland JC, Meyers RA (2008) Anatomy and histochemistry of hindlimb flight posture
in birds. I. The extended hindlimb posture of shorebirds. J Morphol 269:967-
Midtgård U (1989) Circulatory adaptations to cold in birds. In: Bech C, Reinertsen
RE (Hrsg) Physiology of Cold Adaptations in Birds: pp 211-
Necker R (2006) Specializations in the lumbosacral vertebral canal and spinal cord
of birds: evidence of a function as a sense organ which is involved in the control
of walking. J Comp Physiol A: 439-
Necker R (2010) Stehen der Vögel auf einem Bein: Mechanismen und mögliche Funktionen
-
Randler C (2007) Foot preferences during resting in wildfowl and waders. Laterality
12: 191-
Rattenborg NC (1999) Half-
Schaller NU, Herkner B, Villa R, Aerts P (2009) The intertarsal joint of the ostrich
(Struthio camelus): Anatomical examination and function of passive structures in
locomotion. J Anat 214: 830-
Steen I, Steen JB (1965) The importance of the legs in the thermoregulation of birds.
Acta physiol. scand. 63: 285-
Stiefel A (1979) Ruhe und Schlaf bei Vögeln. Neue Brehm-
Stolpe M (1932) Physiologisch-
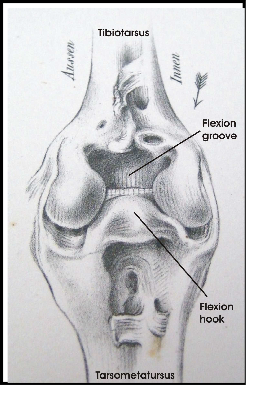
groove
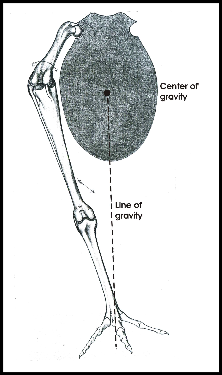

Fig. 4: Gray goose standing on one leg
The most detailed study on the functional organization of hindlimbs of birds, which also deals with the problem of standing on one leg is by Stolpe (1932). Fig. 6 shows a parasagittal section through the lateral condylus of the intertarsal joint of the flamingo (Phoenicopterus ruber). There is a process in the tarsometatarsus which fits into a groove of the condylus of the tibiotarsus in the fully extended joint (see arrows in Fig. 6). Although this looks like a locking mechanism, Stolpe (1932) claims that it only prevents the joint from overstretching. Similar specializations are found in cranes and storks but not in herons (Stolpe 1932).
The possible function of standing on one leg
There are many speculations but few experimental studies or quantitative behavioral observations why birds stand on one leg. A couple of possible functions are published in Flamingo file (1991). The suggestion that there might be a dependence of standing on one leg on unihemispheric sleep, which occurs in some birds (Rattenborg 1999), has been disproved recently (Anderson et al. 2018). Two possible functions which have been tested by quantitative observations in recent publications (Anderson & Williams 2009; Bouchard & Anderson 2011; Anderson & Laughlin 2014) will be dealt with in detail: a thermoregulatory function and relaxation of muscle fatigue in the retracted leg.
Thermoregulatory function. Sleeping positions and the occurrence of sleeping on one
leg among many orders of birds are described in detail by Stiefel (1979). When sleeping
while standing on one leg the head is often hidden in the plumage on the back (Fig.
9). The head is usually positioned on the side of the ground foot or above the center
of gravity. This supports a stable balance. The retracted leg is hidden in the breast
feathers or under the wing. Hiding non-
Reduction of muscle fatigue of the retracted leg. Humans tend to put the body weight
on one leg when standing for longer times. This serves to reduce muscle fatigue in
one leg. Accordingly, Clark (1973) suggested that standing on one leg in birds may
serve a similar function. However, retraction of one leg needs muscle activity. This
is confirmed by an own observation of a resting nile goose (Alopochen aegyptiacus)
whose retracted leg dropped again and again. However, when sleeping with its head
on the back and with closed eyes, the leg was continuously hidden in the plumage.
That leg retraction needs energy is supported by the casual own observation of a
woolly-
Humans of some tribes which use to rest on one leg (e.g. Australian Aborigines or African Bushmen) the retracted leg rests on the supporting leg and equilibrium may be stabilized by leaning on a stick. A casual own obervation shows that storks may use the same technique (Fig. 12).
The question of reduction of muscle fatigue was addressed by Anderson & Williams (2009) in flamingos by measuring the latency of initiating a forward movement. This latency was longer following resting on one leg as compared to the latency when resting on two legs. The authors conclude that this result discounts the possible function of reducing muscle fatigue or enhancing predatory escape.
In most bird species there is no preference of the side (left/right) they use for standing on one leg (Randler 2007; Anderson & Williams 2009; Anderson & Laughlin 2014) and there is an about equal use of left and right leg in individual flamingos (Anderson & Williams 2009). However, it would be interesting to know whether there is a regular shift from one leg to the other leg in order to reduce muscle fatigue in both legs. However, it is not clear whether a reduction of muscle fatigue of the supporting leg or the retracted leg is intended. Given that retracting one leg is energy consuming (see above) one might assume that changing the retracted leg may serve reduction of muscle fatigue of the retracted leg.
Fig 10B: Mute swan on the water with left leg stretched backwards
Fig. 12: Woolly-
The legs of birds are an important site of heat exchange (Steen & Steen 1965; Dawson
& Whittow 2000). In a warm environment heat of the body is dissipated via the legs.
In cold ambient temperature blood supply to the legs is reduced and there is a counter-
In recent investigations it was shown that flamingos spend more time standing on one leg in the water (facilitates heat loss) as compared to standing on one leg on the ground. Furthermore, flamingos stand more often on one leg at cool temperatures (Anderson & Williams 2009; Bouchard & Anderson 2011; Anderson & Laughlin 2014). These quantitative behavioral observations support a thermoregulatory function of standing on one leg. There are, however, observations which show that in the range of 8 to 19 °C, i.e. at low temperatures, the incidence of standing on one leg is reduced (Harker and Harker 2010). The authors argue, that the birds tend to rest/sleep only at higher temperatures. For a detailed discussion of the temperature/rest/sleep topic on standing on one leg see Anderson 2016.
Standing or resting on one leg does not necessarily mean an inactive state. Birds
may practice intense preening while standing on one leg (Fig. 10A). Even aggressive
behavior to neighbors was seen in flamingos while standing on one leg (own observations).
Often resting birds just lift one leg partially without hiding it in the plumage
(see Fotos). In preliminary own observations at the zoo of Dortmund it is shown that
lifting one leg partially occurs more often at higher temperatures (27 C) than at
lower temperatures (20 C). On the other hand complete retraction of one leg was
observed more often at the lower temperatures (Fig. 11). While hiding the non-
Fig. 11A: Sleeping flamingos: Occurence of standing on both legs (no leg retracted), with one leg retracted partially and one leg retracted completely. Blue column: 20 °C (observed in 2008); red column. 27 °C (observed in 2013).
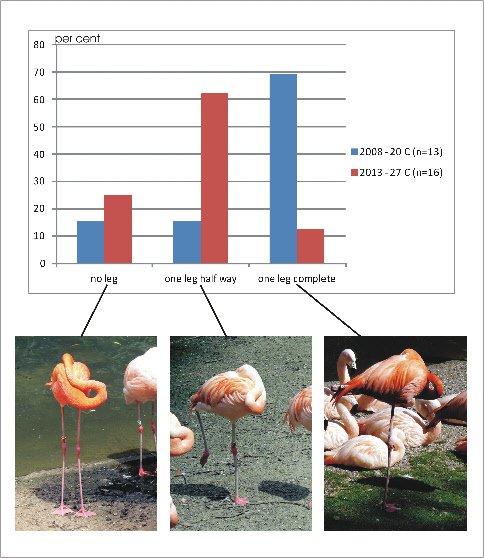
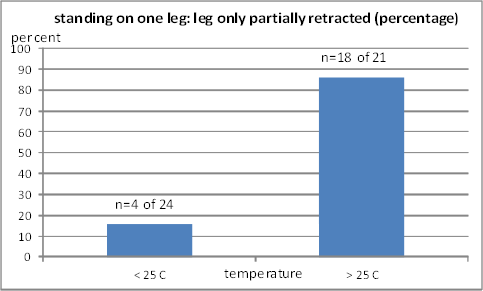
Fig. 11B: Percentage of standing on one leg with the leg retracted only partiallly. Left column: two observations (20 and 23 °C); right column: two observations (27 and 31 °C)
According to Stolpe (1932) there are specializations in the hip joint of long-
In a recent study with flamingo cadavers (Chang & Ting 2017) it was found that standing
on one leg as shown in Fig. 3 (adduction of about 20 degrees from the vertical) results
in a very stable support of the body. Fig. 5 shows schematically the experiments
performed by Chang and Ting. In a resting bird the femur is oriented nearly horizontally,
i.e. the hip joint is maximally flexed. The knee joint forms an angle of about 90
degrees (Fig. 5, 1) which is due to a tibial crest (Baumel and Witmer 1993) at its
maximal extension: downward pressure rostral to the knee does not result in further
extension (Fig. 5, 2). Flexion of the knee joint is still possible as can be seen
from a backward tilt of the body during caudal downward pressure (Fig. 5, 3). Altogether
this results in a stable position of the body since the center of mass is located
rostral to the knee joint („unidirectional stay apparatus“ after Chang and Ting).
Further experiments of Chang and Ting (2017) showed that the body remains quite stable
when simulating a one leg stance with an adducted leg (Fig. 5, 4) wheras it becomes
unstable when simulating a two-
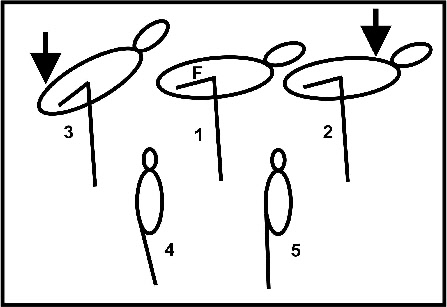
Fig. 5: Sketch of the experiments performed by Chang und Ting (2017).
Upper row: pressure exerted rostral to the knee (arrow) has no effect on body position
(2). Pressure exerted caudally results in a backward body tilt (3). F -
Lower row: normal one-
There are other specializations in the intertarsal joint of long-
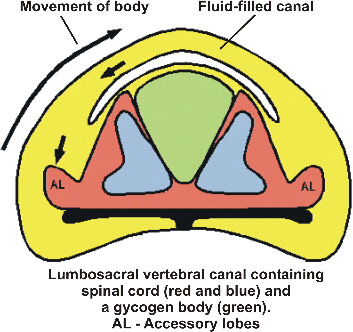
Sensory control of keeping balance when standing on one leg
Keeping balance normally involves several sensory systems: the sense organ of equilibrium located in the inner ear (labyrinth), deep receptors located in muscles and joints as well as the eye. In birds there is an additional sense organ of equilibrium in the lumbosacral region of the vertebral column (Fig. 8; Necker 2006). This sense organ acts directly on the motor system of the legs. It has been shown that the labyrinth has only a weak influence on the motor system in resting birds, so this lumbosacral organ may be used primarily when moving or resting on the ground. Preening on one leg (see Fig. 9) with vigorous movements of the head would impose heavy demands on the function of the labyrinth. Furthermore, when sleeping on one leg with closed eyes this sense organ is also no more available for controlling balance.












